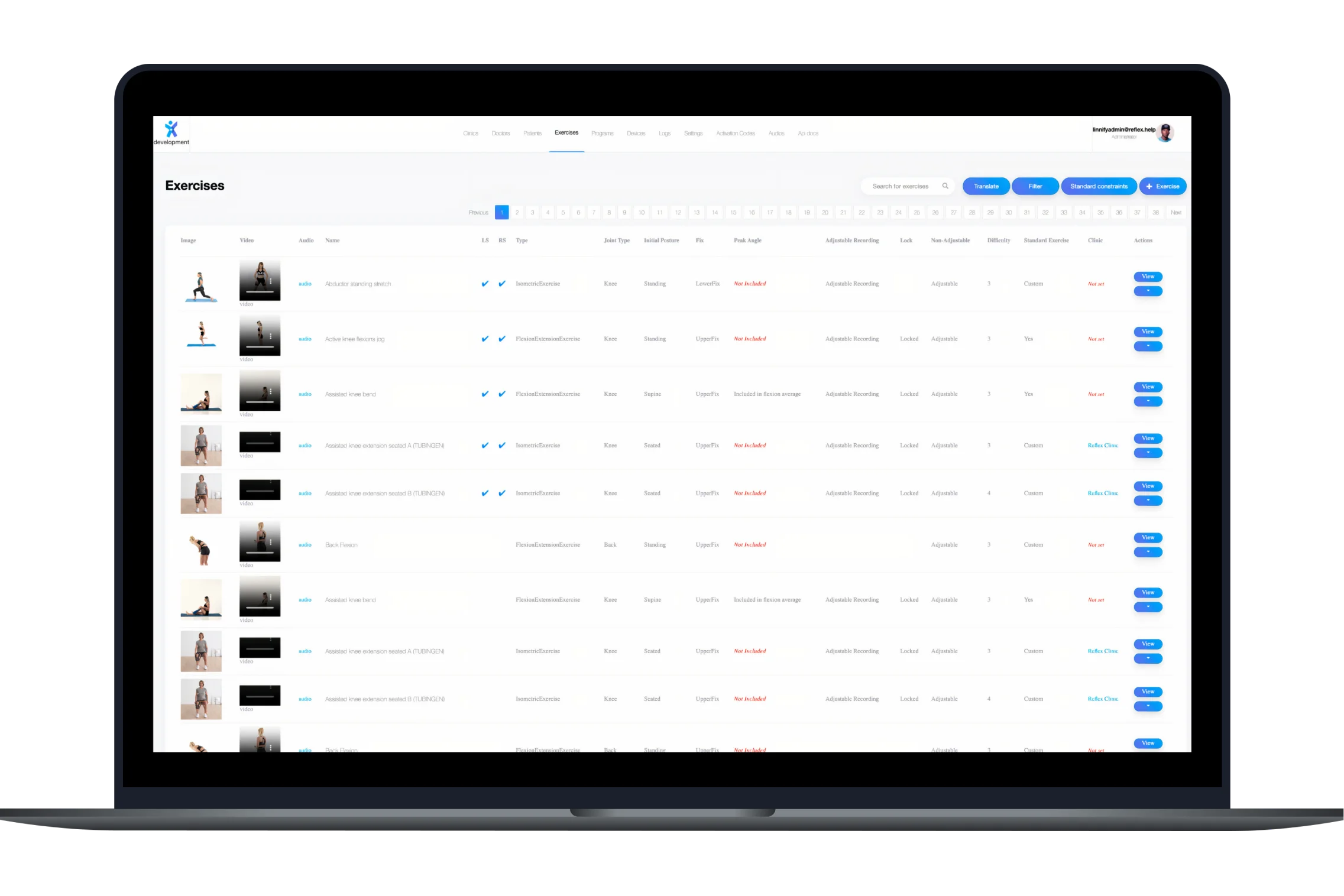
re.flex is a digital MSK (musculoskeletal) therapy assistant using motion sensors and real-time feedback to guide patients through clinically validated recovery exercises for the knee, hip, and lower back.
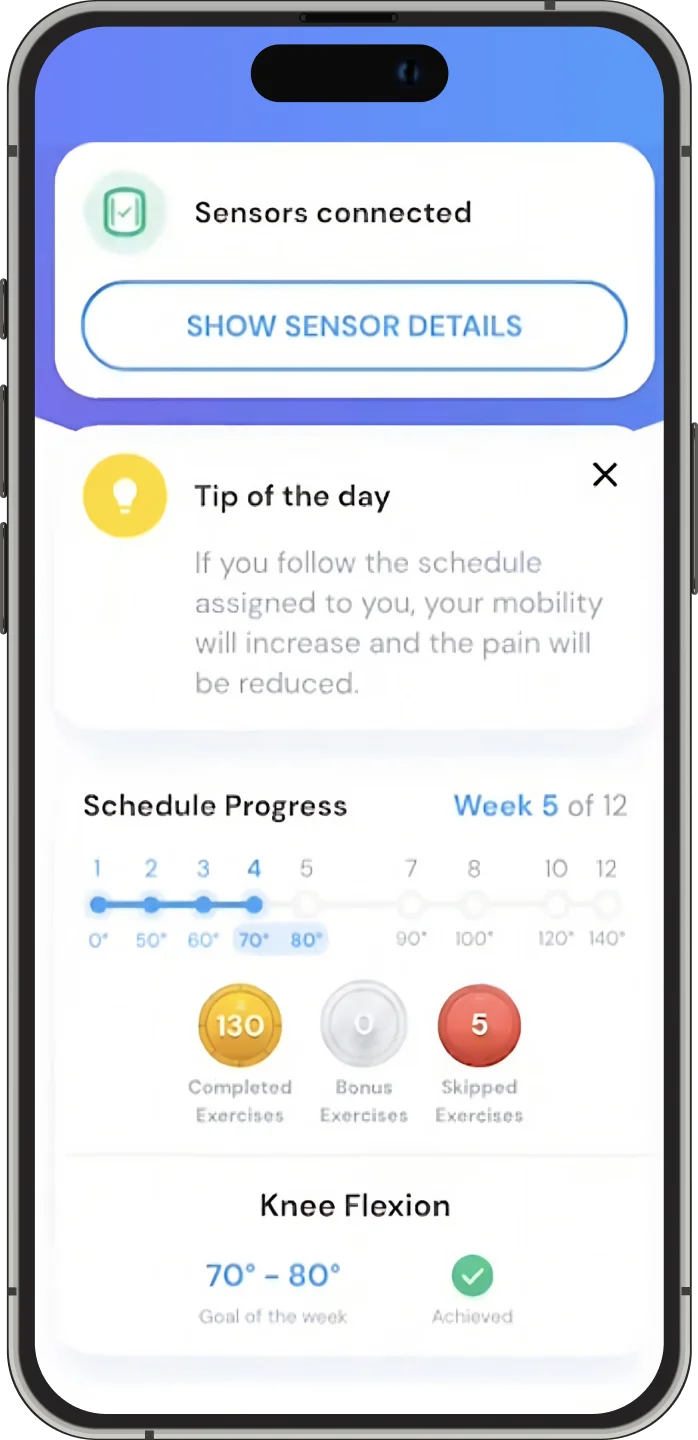
The system includes 3D movement visualizations, instant form correction, and sensor-based accuracy. By combining precision hardware with guided software, re.flex empowers patients to complete therapy programs remotely, safely and effectively.
Building digital therapeutics for the regulated European health market requires strict adherence to compliance, security, and stability standards.
German healthcare system.
Developed specifically for German DiGA requirements, ISO 27001 information security standards, and CE medical device certification for European market expansion.

re.flex faced two mission-critical obstacles:
1. Overcoming tight penetration testing requirements, which blocked entry into clinical trials
2. Delays in launching on Android, which prevented patient onboarding for the study
Without solving these fast, re.flex risked losing momentum and the chance to become a DiGA-certified solution.
Additionally, they needed to expand their Android App Store presence to reach the required number of patients for their clinical trial.
Linnify stepped in to provide targeted backend development and security enhancement services to help re.flex unblock their clinical trial path and move toward DiGA certification.
Phase I: Security-First Clinical Trial Enablement
Linnify's expert development team focused on penetration testing, compliance, and Android platform expansion to unlock clinical trial progression and meet German healthcare security standards.
Phase II: Performance-Driven Code Modernization
Implemented comprehensive JavaScript to TypeScript migration with customer satisfaction integration, feedback systems, and structured code refactoring using industry best practices and Linnify's proprietary boilerplates.
Phase III: Advanced Patient Engagement Features
Developed PDF training report generation system providing personalized patient progress documentation throughout their entire rehabilitation journey.
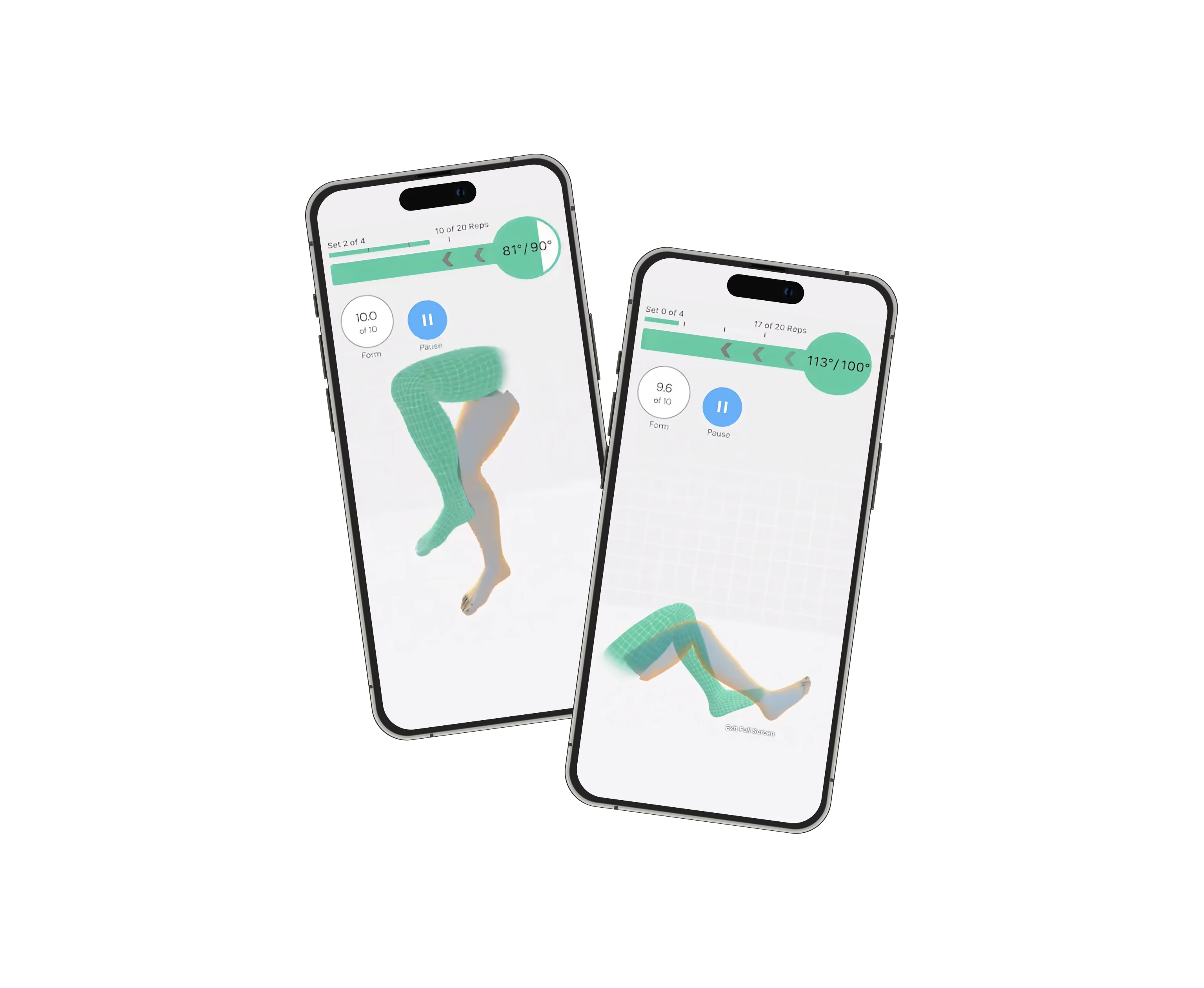
Linnify delivered targeted backend development and security enhancement services for re.flex's digital therapy platform, providing deep expertise in healthcare technology compliance and IoT sensor integration.
Collaboration based on an agile, iterative approach together with re.flex’s CTO, including:

Re.flex achieved breakthrough DiGA certification in 2022, becoming the first Romanian company to successfully navigate Germany's rigorous digital health application approval process.

Linnify’s focused backend development support helped re.flex meet compliance needs, deliver on user-centric features, and achieve a major milestone: obtaining DiGA certification.
The project showcases Linnify’s capabilities in building secure healthcare applications, especially in regulated European markets where stability and documentation are key.
This digital health project significantly enhanced Linnify's expertise in healthcare regulatory compliance, medical device integration, patient data security, and multi-phase product development for complex healthcare technology solutions.


re.flex is a digital physical therapy assistant that uses IoT motion sensors and real-time feedback to guide patients through MSK (musculoskeletal) rehabilitation exercises. It’s designed for patients recovering from knee, hip, or lower back injuries.
re.flex combines real-time 3D movement visualization with IoT sensors to offer accurate form correction and guided therapy. It also meets rigorous compliance standards, including DiGA, ISO 27001, and CE medical device certification.
re.flex was blocked from entering clinical trials due to penetration testing issues and lacked Android deployment capabilities, putting their DiGA certification at risk and slowing patient onboarding.
Linnify provided backend development, security enhancements, TypeScript migration, Android deployment support, and patient reporting systems to unblock clinical trials and improve platform reliability.
DiGA is Germany’s official certification for digital health applications. Achieving DiGA status allows a product to be prescribed by doctors and reimbursed through health insurance—critical for healthcare market access in Germany.
re.flex became the first Romanian company to earn DiGA certification, passed clinical trial security requirements, launched Android capabilities, and added personalized training reports and satisfaction tracking.
It showcases Linnify’s deep expertise in regulated healthcare product development, especially in security, IoT sensor integration, patient data systems, and scalable backend architecture.